
In this model n = ∞ corresponds to the level where the energy holding the electron and the nucleus together is zero.

Which metal cation was observed to emit radiation with the longest wavelength?Ĭompared to the other metals studied, did the radiation emitted by this metal cation have.What evidence is there that the colors observed in the flame tests are due to the metals, and not the nonmetals in the compounds tested?.The electrons in these metals then made transitions from (low, high) energy levels to (low, high) energy levels, resulting in the (absorption, emission) of energy as (electricity, heat, EM radiation). The metals were then in the (ground, excited) state. Background The Rydberg Formula for the wavelength of the radiation emitted in atomic energy level transitions is 1 ZR1 - 1) Infinal ninitial where R 1.097x10'm-1, Z is the atomic number number of protons) of the element, Nfinal is the principal quantum number. When this occurred, electrons made transitions from (low, high) energy levels to (low, high) energy levels. Lab 9 Atomic Line Spectra and PHYS 112 Atomic Structure Name: I. When placed in the flame, the metals then (absorbed, emitted) energy as (electricity, heat, EM radiation). Name the colors of visible light beginning with that of highest energy (shortest wavelength). In this experiment, the metal cations in the solutions were initially in the (ground, excited) state. Chemistry Chemistry questions and answers Atomic Spectra and Atomic Structure 12 Pre-lab Questions Before beginning this experiment in the laboratory, you should be able to answer the following questions. Complete the following paragraph by circling the correct responses:.In fact, flame tests were used to identify elements long before the invention of modern techniques, such as emission spectroscopy. As many elements will still produce distinctive colors under such conditions, simple flame tests can be used to identify these elements. This one color results from a combination of all lines of the emission spectrum, in proportion to their intensities. For example, helium gas when excited by an electrical discharge emits light that appears an orange- peach color. To the naked eye, when an element is vaporized in a flame (or an electrical discharge)the emission spectrum will appear to be just one color. Unfortunately, techniques more sophisticated than those used in this lab are required to obtain such line spectra.

#Atomic spectra and atomic structure pre lab answers pdf#
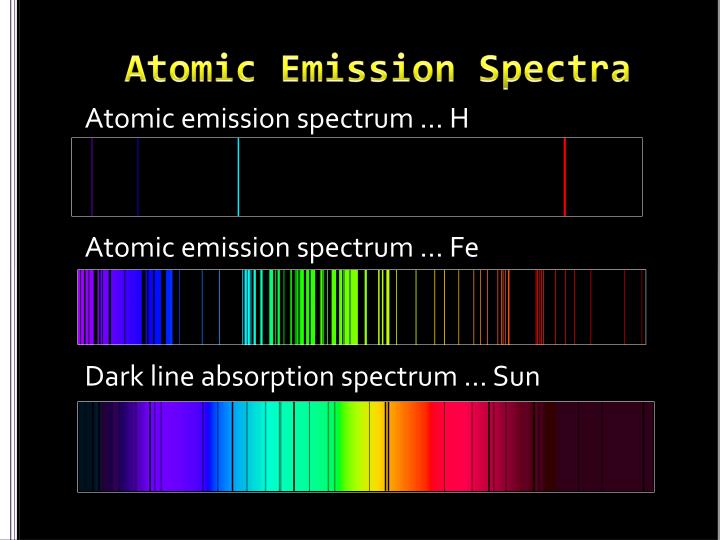
For example, the line spectra shown below for the elements helium and carbon are clearly quite different (colors can be seen in the PDF document on-line). The result is called a line emission spectrum, and can serve as a ‘fingerprint’ of the element to which the atoms belong. If emitted photons are in the visible region of the spectrum, they may be perceived as lines of different colors (note that photons outside the visible spectrum may also be emitted, but cannot be seen). The spacing between energy levels in an atom determines the sizes of the transitions that occur, and thus the energy and wavelengths of the collection of photons emitted. However, when electrons subsequently return from higher energy levels to lower energy levels, energy is released predominantly in the form of electromagnetic radiation. The energy absorbed could be in the form of heat (as in flame tests), or electrical energy, or electromagnetic radiation. So, how does electromagnetic radiation relate to flame tests? Well, when an atom or ion absorbs energy, its electrons can make transitions from lower energy levels to higher energy levels. Other examples of electromagnetic radiation include X-rays, ultraviolet light, infrared light, microwaves and radio waves. Differences in the wavelengths of visible light are manifested as different colors, shown in the color spectrum below (colors can be seen in the PDF document on-line). Visible light is the most familiar example of electromagnetic radiation.


 0 kommentar(er)
0 kommentar(er)
